Radiation Interactions - Physics
Introduction
This page looks at the interactions of the radiations of principal interest namely alpha, beta, gamma and neutron and then briefly discusses which nuclei decay.
The important thing to take from this page is that ionising radiations, as the name suggests, ionise the material be it air, water, flesh, whatever, they pass through and leave ion pairs along their tracks. These ion pair consist of a free electron and an ionised atom or molecule. Later we will learn that these cause chemical changes that can lead to injury in living tissue.
Alpha Particles
Alpha particles are produced by the decay of heavy unstable nuclei or in ternary fission. Natural alpha emitters include uranium, thorium and radium.
Nuclear reactors also produce transuranic elements including neptunium, plutonium and americium.
The alpha particle is a relatively heavy entity (4 atomic mass units) being composed of two neutrons and two protons. It has a charge of +2e (where e is the electron charge of 1.602 x 10-19 Coulombs). Alpha particles typically have energies between 3 and 7 MeV and travel at speeds of 15,000 km·s-1 (5% of the speed of light) which is slow in comparison to beta and gamma radiation. Because of its charge the alpha particle interacts strongly with the medium it is travelling in (mediated by the Coulomb force) causing dense ionisation. Each coulomb interaction costs the alpha particles a small fraction of its energy. Therefore its deflection is almost negligible and it travels in a almost straight paths in matter, losing energy continuously through a large number of collisions. Because it loses a lot of energy over a short path length (in the jargon it has a high linear energy transfer (LET)) it runs out of energy quickly and has a short range in matter. When the alpha's energy is reduced to a low enough level it can capture an electron which reduces its charge and the rate of energy loss. The capture of a second electron neutralises it and turns it into a helium atom.
Coulomb force: There is a force between charges that causes attraction of unlike charges and repulsion of alike charges. This force works at a distance significantly greater than the atomic radius and acts to slow down moving charged particles by transferring some of the kinetic energy of the particle to the atoms they interact with.
Alpha particles are readily stopped due to their charge and mass. This has three main implications:
- Alpha particles are not generally an external hazard as they are stopped by the dead (epidermal layer) layer of skin, which covers most of the body. The exception is the lens of the eye which has no epidermal layer.
- The range of alpha particles (which is energy dependent) is typically a few centimetres (3 - 5cm) in air.
- Internal to the human body alpha particles are more damaging per unit of energy than gamma rays and beta particles as their shorter stopping distance means that they deposit a lot of energy within a smaller volume. This means that they are capable of causing more intense damage which reproducing cells find harder to repair. For a given energy there is more radiological risk associated with an alpha particle than most other types of radiation.
Beta particles
Beta particles are electrons (β or β-) or positrons (β+) which are ejected from the nucleus at relativistic speeds (>75% of the speed of light) and they are
often emitted in conjunction with gamma rays. They are relatively light (1/1835 atomic mass units) and have a charge of -e (electron) and +e (positron). They do not interact as
strongly as the alpha particle but when they do interact they can be scattered over a wider angle and lose a larger fraction of their energy in each interaction.
Beta particles are produced by a neutron converting to a proton, an electron and a neutrino (beta+ from a neutron converting to a proton, a positron and a neutrino). Particles from a particular decay have a range of energies up to a characteristic maximum energy (the balance of energy being carried of by the ghostly particle called a neutrino). The resulting daughter nucleus following a beta decay is usually in an excited state and emits characteristic gamma radiation in falling to its ground state.
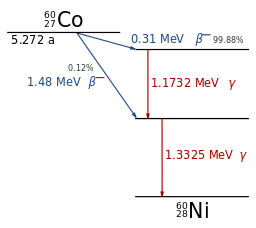
(See diagram), an example of beta decay is 60Co (colbalt-60). This decays to 60Ni (nickel-60) with a half-life of 5.27 years. Most of the time (99.88%) the colbalt-60 decays to an excited state in nickel-60 which then falls to the ground state giving off two gamma photons (energy 1.1732 MeV and 1.3325 MeV). Occassionally (0.12% of the time) the decay reaches a lower excited state and decays via the one gamma at 1.3325 MeV. The first decay mode gives a beta particle with a maximum energy of 0.31 MeV and the second a beta particle with a maximum of 1.48 MeV.
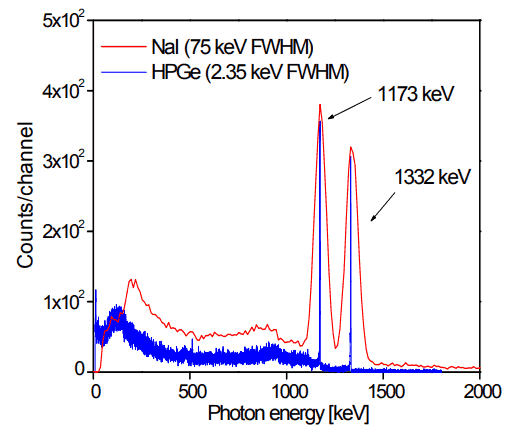
With its two gamma rays, Co-60 is easy relatively easy to identify on a gamma spectrometer. See figure from Jonah Messinger.
Co-60 is a problem to the nuclear industry. It is produced in reactors by neutron activation of cobalt-59 which is found in many specialist steels used in reactors. It produces two gamma photons and contributes a significant fraction of the radiation dose from recently used fuel. Its half life of just over five years means that "delay and decay" can be used to manage it. This entails keeping it safely contained for 25 - 50 years after which its contribution to the doserate is much reduced.
The range of beta particles in air is generally ~15 cm but for high energy beta particles (2 MeV) it may be up to 2 m. Beta particles are decelerated by electromagnetic interactions and as a result may give off bremsstrahlung (breaking) x-rays. Low atomic number materials (such as water and plastic) are preferred as beta shields because they produce less damaging X-ray radiation than high atomic number (high z) materials.
Beta radiation can penetrate the outer layers of skin (epidermal) and damage the living layer below (the dermis and hypodermis). It can cause skin burning (erythema) at high levels of contamination and, like alpha contamination, is more dangerous if the source is taken into the body.
Interaction of charged particles (alpha and beta particles) with matter
Charged particles, including beta particles and alpha particles, interact with the human tissue they are passing through via electrostatic interactions (the coulomb force
between the radiation's charge and the atom's outer electrons).
They lose energy while the surrounding tissue gains energy by excitation and ionisation of atoms.
Excitation - an electron is promoted to a higher energy state where it resides for a time before deexciting and giving out a photon, or photons, of energy.
Ionisation – an orbital electron is removed from an atom resulting in an electron and a positively charged ion, collected called an ion pair.
As the alpha particle has a larger charge than the electron it interacts more with the material it is travelling through, loses energy more quickly, travels less far and causes many more ion pairs to be created per length of track. As it is much heavier than the electron and therefore has a greater momentum, it deviates less from its path in each interaction than an electron which bounces all over the place.
Charged particles also lose energy as Bremsstrahlung emission when they are deflected by the electrostatic field of an atoms. This is more important for electrons and positrons as they experience greater acceleration.
Bremsstrahlung radiation is electromagnetic in nature (similar to X-rays) and is produced when a beta particle is deflected by an atom and loses
kinetic energy in the process. This energy is expressed as a photon for which hν = Ei - Ef. where
ν frequency of photon radiation (Hz = s-1)
h Planck's constant (6.62607015 × 10−34 J⋅Hz-1)
Ei Energy of beta particle before the collision
Ef Energy of beta particle after the collision
This produces a range of energies up to the energy of the beta particle before the collision.
Neutron
The neutron, is simply that, a neutron; one of the sub-atomic particles. Because it has no charge the neutron does not experience the Coulomb force and will only interact with atomic nuclei by direct collision. As it does not interact continuously it can travel some distance without a change in direction or loss of energy. Its range in material is very much greater than that of the charged particles and it deposits energy non-uniformly. For fast neutrons, such as those produced in fission, materials with light atoms (such as water or polythene, which both contain hydrogen) are more effective shields as each collision with a light nucleus takes a larger fraction of the energy from the neutron.
The interactions of neutrons with matter
Neutrons have no electrostatic charge. To interact they have to physically collide with nuclei in the material they are passing through. This can result in the neutron scattering (bouncing off in a different direction) or combining with the target nucleus. The probability of interaction depends on the number of neutrons involved, the number of target atoms involved and the probability of an interaction with a nucleus (expressed as a cross-section because it has dimensions of area. The common unit is the barn which is 10-24 cm-2).
The probability (cross section) has a very strong depenancy on the energy of the neutron and the identitiy of the target nuclides.
The energy of neutrons tend to be divided into energy bands although these have no formally accepted value. We can use:
- Thermal (0.025 eV)
- Slow (<10 eV)
- Intermediate (10 eV - 100 keV)
- Fast (>100 keV)
The neutrons produced in a nuclear reactor have significant kinetic energy; they are fast neutrons. They are moderated (slowed) due to multiple scattering events. Once they reach thermal energies they are absorbed by a target nucleus.
There are several types of collision to consider:
- Elastic scattering in which the kinetic energy is conserved but shared between the, now slowed, neutron and the recoiling nucleus;
- Inelastic scattering where some of the kinetic energy is used to excite the recoiling nucleus (which will produce one or more gammas when it returns to the ground state);
- Charged particle reactions where the neutron is absorbed and a different charged particle is emitted.
Collisions with light nuclei such as hydrogen remove more of the neutron's kinetic energy than collisions with heavier nuclei per collision.
The result of these reactions is to scatter the neutron, reduce its energy and produce a field of gamma radiation and charged particles. These secondary radiations then interact with the medium they are in and can ionise it. For this reason neutrons are called indirectly ionising radiation.
Gamma
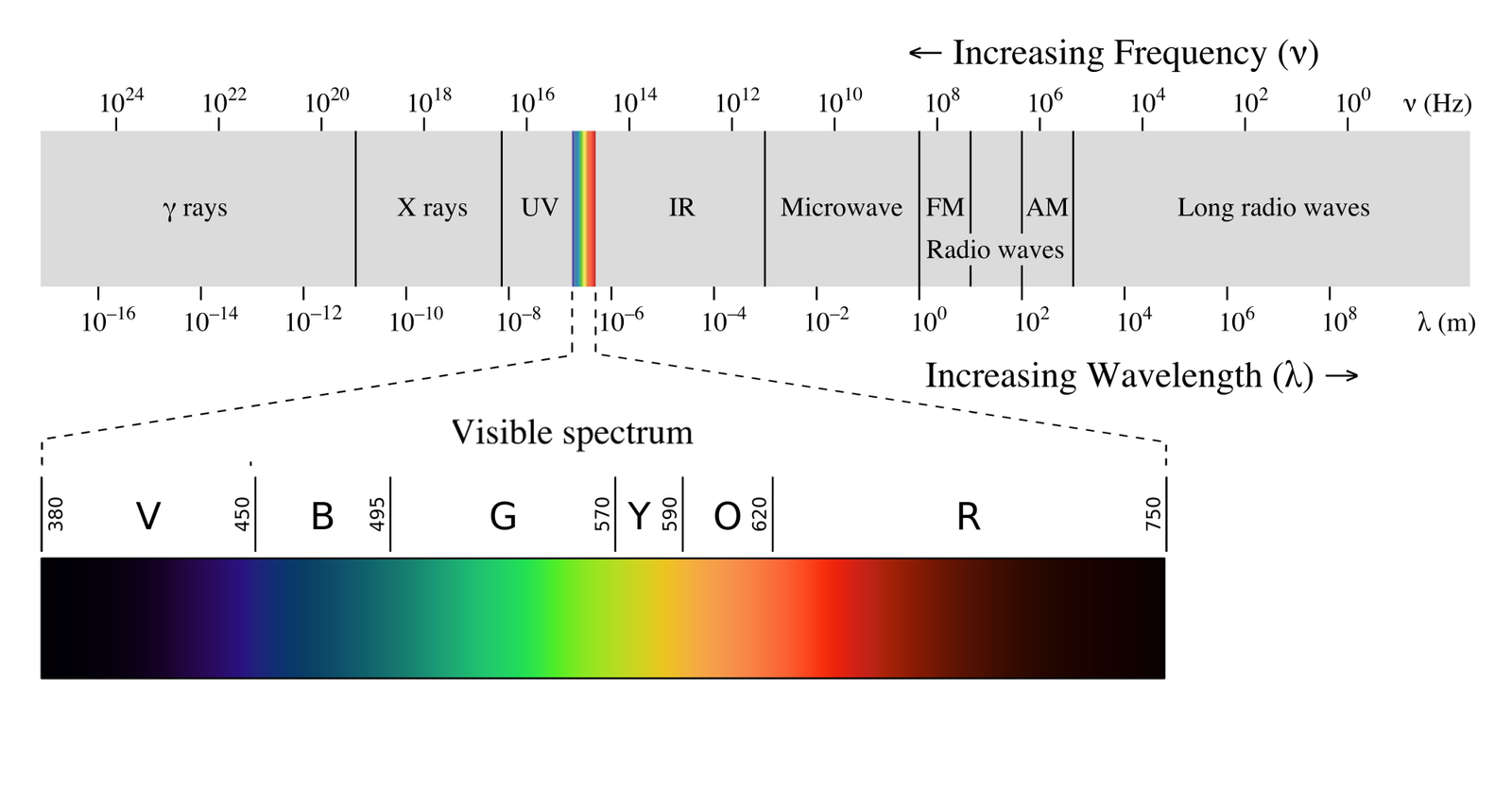
Gamma radiation is an electromagnetic radiation like light, x-rays and radio waves but is higher in energy. Gamma rays travel at the speed of light and for nuclear decay energies generally range from 100 keV to 10 MeV and are typically ~1 MeV.
Gamma radiation has a relatively long range in material and only dense materials such as steel or lead, or a significant path-length in other materials such as water, provide an effective shield. Gamma radiation can deliver a significant dose to internal organs without the source being taken into the body.
Gamma interactions with matter
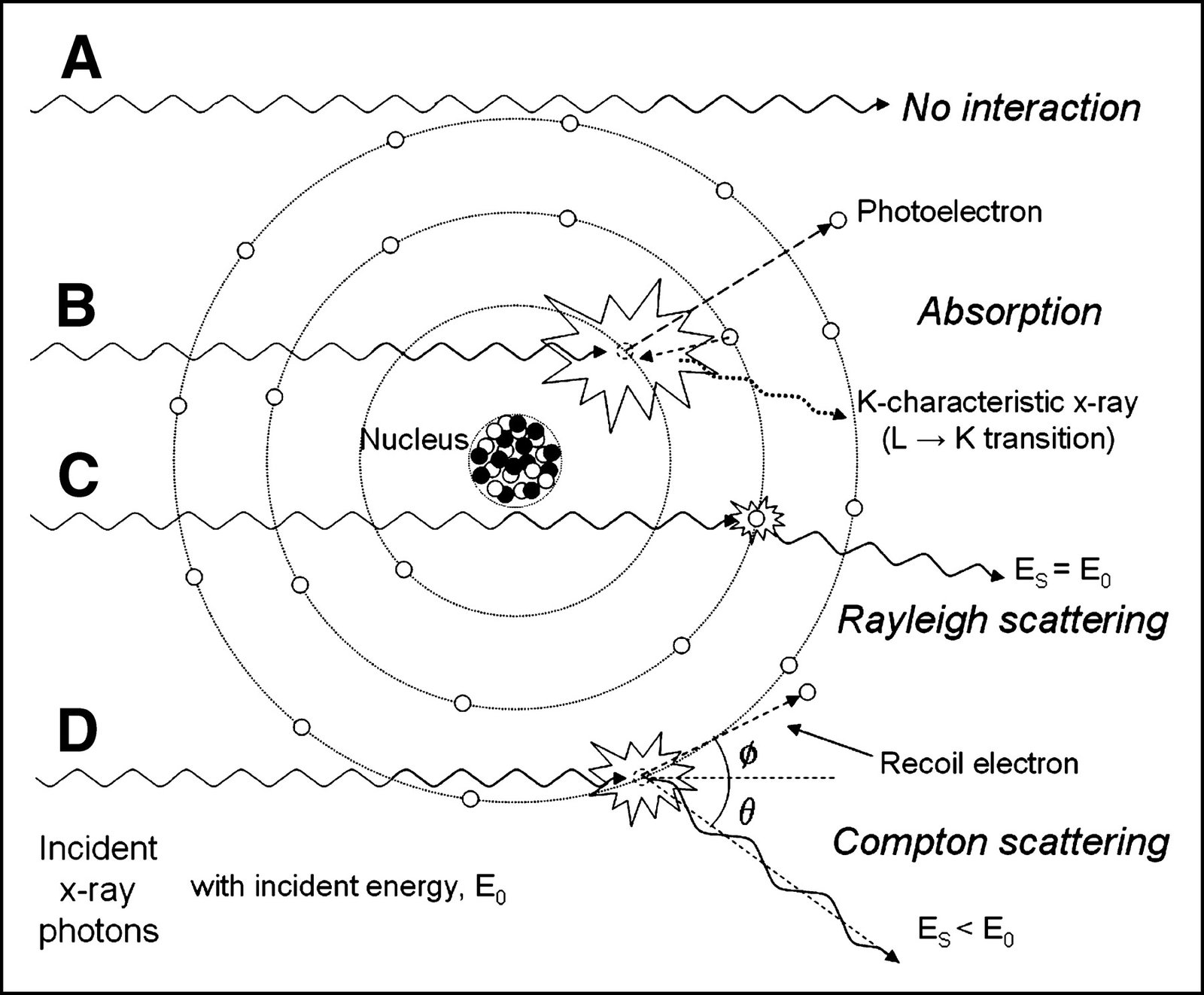
Gamma radiation interacts with material in three main ways:
- The photelectric effect - in which the gamma is absorbed and an electron is ejected from an atom
- Comptom scattering - in which the gamma loses energy and is deflected
- Pair production - in which an electron/positron pair are produced and the gamma absorbed
Photoelectric effect If the photon energy is a bit bigger than the binding energy of an atomic electron the gamma can be absorbed by the atom, ejecting an electron (which then has kinetic energy equal to the photon's orginal energy minus the electron's binding energy). The atom will then decay back to its ground state giving off one or more low energy photons.
Comption scattering involves a gamma photon in an elastic scattering event with an atomic electron. This produces a free electron (which loses energy by ionising and exciting atoms along its path) and a lower energy gamma photon moving in a different direction. This occurs more frequently when the initial gamma energy is greater than the binding energy of the electron to the atom.
Electron-positron pair production can occur when the gamma energy exceeds 1.022 MeV. The gamma is absorbed and a positron and electron produced. These each cost 0.511MeV to produce (E = mc2) and each has kinetic energy equal to half of the remaining energy. Both the electron and positron ionise and excite along their path before the electron is captured by an atom and the positron annililated by collision with an electron yielding two 0.511 MeV photons.
Less important for our purposes is Rayleigh scattering in which a photon interacts with the electromagnetic field of the whole atom and scatters with very little change in its energy. (Although unimportant in this context, Rayleigh scattering does cause the sky to be blue).
Because of the manner in which gamma rays interact with material their range can be significant. Rather then being limited in range, they experience exponential attenuation leading to the concepts of half value layers of material that remove half of the original gammas. Like neutrons, Gamma photons are indirectly ionising radiations in that it is the product of their various primary interactions that cause the ionisation.
Which nuclei decay?
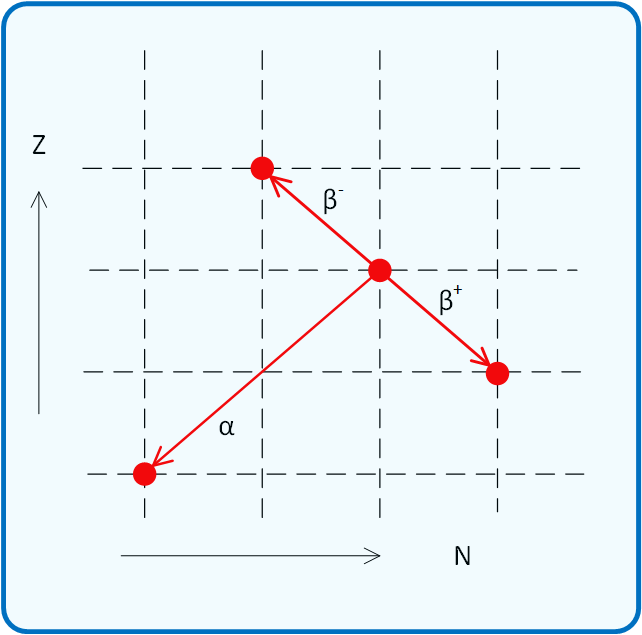
The nuclei of atoms are built of neutrons and protons. The number of protons in a nucleus (z) determines which chemical it is. The sum of the number of neutrons (n) and protons is the mass number (a) of the nucleus.
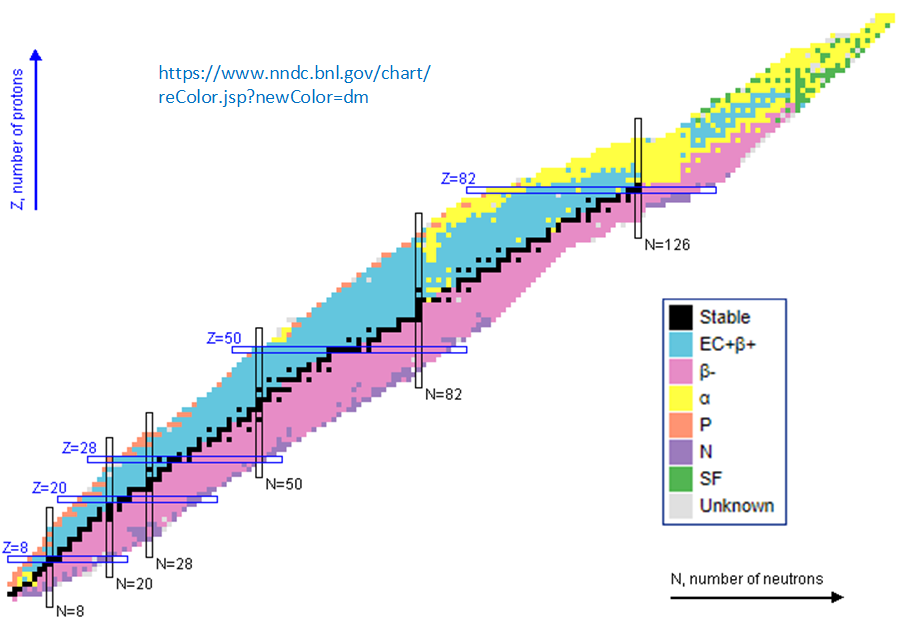
If you plot some nuclear properties of the nuclei on grid of number of neutrons against number of protons you can determine some interesting and useful trends. For example from the
NZ decay diagram, which colour codes the most likely decay mode of every known type of nuclei on the NZ grid (see below) we see a "valley of stability"
(the black boxes denote stable isotopes) that approximately follows the N=Z line for light
nuclei but leans towards a higher proportion of neutrons for heavier nuclei, nuclei below this line (relatively neutron rich) tend to beta decay which moves them one step to the
left and one step upwards, towards the valley of stability; nuclei above this line (relatively proton rich) tend to beta+ decay, one step down, one step to the right,
and very heavy nuclei tend to alpha decay (two steps down, two to the left). These three decay modes are often accompanied with gamma photons as the resultant nuclei drops to its ground state.
Decay chains
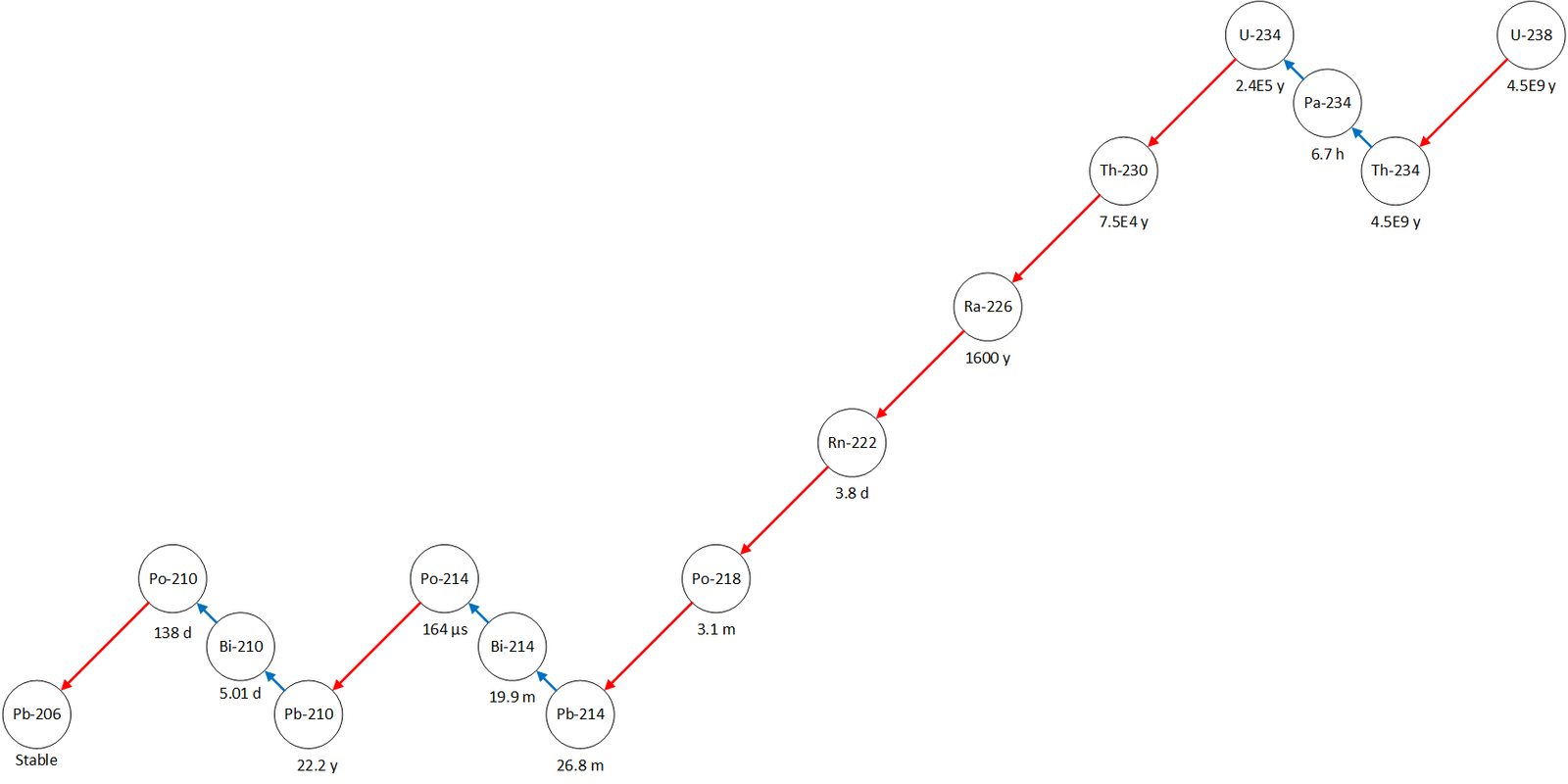
Not all decays result in a stable nucleus. Some lead to another decay giving us decay chains.